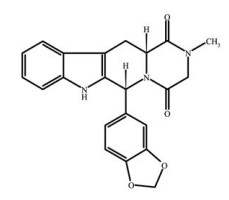
Tadalafil is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise ability. Studies establishing effectiveness included predominately patients with NYHA Functional Class II - III symptoms and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (23%).
Tadalafil is an inhibitor of phosphodiesterase type 5 (PDE5), the enzyme responsible for the degradation of cyclic guanosine monophosphate (cGMP). Pulmonary arterial hypertension is associated with impaired release of nitric oxide by the vascular endothelium and consequent reduction of cGMP concentrations in the pulmonary vascular smooth muscle. PDE5 is the predominant phosphodiesterase in the pulmonary vasculature. Inhibition of PDE5 by tadalafil increases the concentrations of cGMP resulting in relaxation of pulmonary vascular smooth muscle cells and vasodilation of the pulmonary vascular bed.
Studies in vitro have demonstrated that tadalafil is a selective inhibitor of PDE5. PDE5 is found in pulmonary vascular smooth muscle, visceral smooth muscle, corpus cavernosum, skeletal muscle, platelets, kidney, lung, cerebellum, and pancreas
In vitro studies have shown that the effect of tadalafil is more potent on PDE5 than on other phosphodiesterases. These studies have shown that tadalafil is >10,000 times as potent for PDE5 than for PDE1, PDE2, PDE4, and PDE7 enzymes, which are found in the heart, brain, blood vessels, liver, leukocytes, skeletal muscle, and other organs. Tadalafil is >10,000 times as potent for PDE5 as for PDE3, an enzyme found in the heart and blood vessels. Additionally, tadalafil is 700-fold as potent for PDE5 than for PDE6, which is found in the retina and is responsible for phototransduction. Tadalafil is >9,000-fold as potent for PDE5 as for PDE8, PDE9, and PDE10. Tadalafil is 14 times as potent for PDE5 as for PDE11A1 and 40 times as potent for PDE5 as for PDE11A4, two of the four known forms of PDE11. PDE11 is an enzyme found in human prostate, testes, skeletal muscle and in other tissues. In vitro, tadalafil inhibits human recombinant PDE11A1 and, to a lesser degree, PDE11A4 activities at concentrations within the therapeutic range. The physiological role and clinical consequence of PDE11 inhibition in humans have not been defined.
Rakshit is a leading manufacturer and supplier of Active Pharmaceutical Ingredients (APIs) through our manufacturing sites in Hyderabad and Vizag. Our API facilities have been inspected and approved by different international regulatory bodies including the US FDA, Japan PMDA, KFDA, TGA and meet the cGMP standards. We have sustainably evolved as one of the fast growing organization in the pharmaceutical industry in India. To cater to market requirements, we have dedicated our 6 manufacturing facilities to our core business area – APIs ably supported well-equipped Research & Development laboratory both for process improvement and new product development.
We have been successfully supplying APIs all over the globe for more than Two decades. We are capable of executing extensive API requirements for our customers. Rakshit has an experienced team of skilled employees, who are equipped for API manufacture with adequate care. We have a stand-alone R&D center located in Visakhapatnam with State-of-the-art analytical facilities and around 100 employees. We have wide variety of products in diverse therapeutic segments.
Some of the Active Pharmaceuticals Ingredients (APIs) mentioned in the Rakshit’s product list or brochure or in the product list uploaded in Rakshit’s website may be protected by a valid patent in India or in any other countries concerned. However, mentioning of the name of such product(s) in the product brochure or the product list shall not be construed as infringement in any manner whatsoever. Without prejudice to the foregoing, Rakshit’s supply of API being protected by a valid product patent, if any, is intended solely for uses reasonably relating to the research and development, and regulatory submission under any law for the time being inforce, in India, or in a country other than India that regulates the manufacture, use, sale or import of the said product, strictly under the conditions as set forth in Section 107A of the Indian patents Act, 1970, as amended in 2005, or an equivalent provision (Bolar exemption) thereof in a country other than India. The disclaimer is applicable till the expiration of the corresponding product patent(s), if any, in the respective countries concerned. None of the products will be supplied to the countries wherein this could be in conflict with existing laws. However, the final responsibility lies exclusively with the buyer/end customer