Prophylaxis of Organ Rejection in Kidney Transplant: Mycophenolic acid is indicated for the prophylaxis of organ rejection in adult patients receiving a kidney transplant. Mycophenolic acid is indicated for the prophylaxis of organ rejection in pediatric patients 5 years of age and older who are at least 6 months post kidney transplant. Mycophenolic acid is to be used in combination with cyclosporine and corticosteroids.
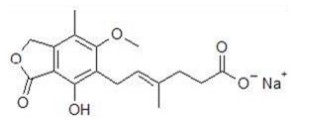
Mycophenolic acid (MPA), an immunosuppressant, is an uncompetitive and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), and therefore inhibits the de novo pathway of guanosine nucleotide synthesis without incorporation to DNA. T- and B-lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, whereas other cell types can utilize salvage pathways. MPA has cytostatic effects on lymphocytes. Mycophenolate sodium has been shown to prevent the occurrence of acute rejection in rat models of kidney and heart allotransplantation. Mycophenolate sodium also decreases antibody production in mice.
Rakshit is a leading manufacturer and supplier of Active Pharmaceutical Ingredients (APIs) through our manufacturing sites in Hyderabad and Vizag. Our API facilities have been inspected and approved by different international regulatory bodies including the US FDA, Japan PMDA, KFDA, TGA and meet the cGMP standards. We have sustainably evolved as one of the fast growing organization in the pharmaceutical industry in India. To cater to market requirements, we have dedicated our 6 manufacturing facilities to our core business area – APIs ably supported well-equipped Research & Development laboratory both for process improvement and new product development.
We have been successfully supplying APIs all over the globe for more than Two decades. We are capable of executing extensive API requirements for our customers. Rakshit has an experienced team of skilled employees, who are equipped for API manufacture with adequate care. We have a stand-alone R&D center located in Visakhapatnam with State-of-the-art analytical facilities and around 100 employees. We have wide variety of products in diverse therapeutic segments.
Some of the Active Pharmaceuticals Ingredients (APIs) mentioned in the Rakshit’s product list or brochure or in the product list uploaded in Rakshit’s website may be protected by a valid patent in India or in any other countries concerned. However, mentioning of the name of such product(s) in the product brochure or the product list shall not be construed as infringement in any manner whatsoever. Without prejudice to the foregoing, Rakshit’s supply of API being protected by a valid product patent, if any, is intended solely for uses reasonably relating to the research and development, and regulatory submission under any law for the time being inforce, in India, or in a country other than India that regulates the manufacture, use, sale or import of the said product, strictly under the conditions as set forth in Section 107A of the Indian patents Act, 1970, as amended in 2005, or an equivalent provision (Bolar exemption) thereof in a country other than India. The disclaimer is applicable till the expiration of the corresponding product patent(s), if any, in the respective countries concerned. None of the products will be supplied to the countries wherein this could be in conflict with existing laws. However, the final responsibility lies exclusively with the buyer/end customer