Reach Out Today!
Plot # 425/3RT, SR Nagar, Hyderabad - 500038, INDIA
info@rakshitdrugspvtltd.com
(91) 40 2370 5066
(91) 40 2381 2709
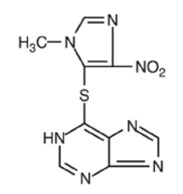
Azathioprine is indicated as an adjunct for the prevention of rejection in renal homotransplantation. It is also indicated for the management of active rheumatoid arthritis to reduce signs and symptoms.
Homograft Survival: The use of azathioprine for inhibition of renal homograft rejection is well established, the mechanism(s) for this action are somewhat obscure. The drug suppresses hypersensitivities of the cell-mediated type and causes variable alterations in antibody production. Suppression of T-cell effects, including ablation of T-cell suppression, is dependent on the temporal relationship to antigenic stimulus or engraftment. This agent has little effect on established graft rejections or secondary responses. Alterations in specific immune responses or immunologic functions in transplant recipients are difficult to relate specifically to immunosuppression by azathioprine. These patients have subnormal responses to vaccines, low numbers of T-cells, and abnormal phagocytosis by peripheral blood cells, but their mitogenic responses, serum immunoglobulins, and secondary antibody responses are usually normal.
Immunoinflammatory Response: Azathioprine suppresses disease manifestations as well as underlying pathology in animal models of autoimmune disease. For example, the severity of adjuvant arthritis is reduced by azathioprine. The mechanisms whereby azathioprine affects autoimmune diseases are not known. Azathioprine is immunosuppressive, delayed hypersensitivity and cellular cytotoxicity tests being suppressed to a greater degree than are antibody responses. In the rat model of adjuvant arthritis, azathioprine has been shown to inhibit the lymph node hyperplasia, which precedes the onset of the signs of the disease. Both the immunosuppressive and therapeutic effects in animal models are dose-related. Azathioprine is considered a slowacting drug and effects may persist after the drug has been discontinued.
Rakshit is a leading manufacturer and supplier of Active Pharmaceutical Ingredients (APIs) through our manufacturing sites in Hyderabad and Vizag. Our API facilities have been inspected and approved by different international regulatory bodies including the US FDA, Japan PMDA, KFDA, TGA and meet the cGMP standards. We have sustainably evolved as one of the fast growing organization in the pharmaceutical industry in India. To cater to market requirements, we have dedicated our 6 manufacturing facilities to our core business area – APIs ably supported well-equipped Research & Development laboratory both for process improvement and new product development.
We have been successfully supplying APIs all over the globe for more than Two decades. We are capable of executing extensive API requirements for our customers. Rakshit has an experienced team of skilled employees, who are equipped for API manufacture with adequate care. We have a stand-alone R&D center located in Visakhapatnam with State-of-the-art analytical facilities and around 100 employees. We have wide variety of products in diverse therapeutic segments.
Some of the Active Pharmaceuticals Ingredients (APIs) mentioned in the Rakshit’s product list or brochure or in the product list uploaded in Rakshit’s website may be protected by a valid patent in India or in any other countries concerned. However, mentioning of the name of such product(s) in the product brochure or the product list shall not be construed as infringement in any manner whatsoever. Without prejudice to the foregoing, Rakshit’s supply of API being protected by a valid product patent, if any, is intended solely for uses reasonably relating to the research and development, and regulatory submission under any law for the time being inforce, in India, or in a country other than India that regulates the manufacture, use, sale or import of the said product, strictly under the conditions as set forth in Section 107A of the Indian patents Act, 1970, as amended in 2005, or an equivalent provision (Bolar exemption) thereof in a country other than India. The disclaimer is applicable till the expiration of the corresponding product patent(s), if any, in the respective countries concerned. None of the products will be supplied to the countries wherein this could be in conflict with existing laws. However, the final responsibility lies exclusively with the buyer/end customer