Reach Out Today!
Plot # 425/3RT, SR Nagar, Hyderabad - 500038, INDIA
info@rakshitdrugspvtltd.com
(91) 40 2370 5066
(91) 40 2381 2709
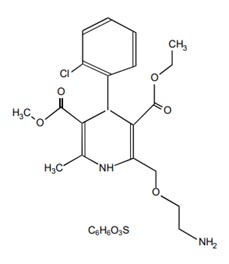
Amlodipine is a calcium channel blocker and may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of:
Hypertension
Coronary Artery Disease
Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.
The precise mechanisms by which amlodipine relieves angina have not been fully delineated, but are thought to include the following:
Exertional Angina: In patients with exertional angina, Amlodipine reduces the total peripheral resistance (afterload) against which the heart works and reduces the rate pressure product, and thus myocardial oxygen demand, at any given level of exercise.
Vasospastic Angina: Amlodipine has been demonstrated to block constriction and restore blood flow in coronary arteries and arterioles in response to calcium, potassium epinephrine, serotonin, and thromboxane A2 analog in experimental animal models and in human coronary vessels in vitro. This inhibition of coronary spasm is responsible for the effectiveness of Amlodipine in vasospastic (Prinzmetal’s or variant) angina.
Rakshit is a leading manufacturer and supplier of Active Pharmaceutical Ingredients (APIs) through our manufacturing sites in Hyderabad and Vizag. Our API facilities have been inspected and approved by different international regulatory bodies including the US FDA, Japan PMDA, KFDA, TGA and meet the cGMP standards. We have sustainably evolved as one of the fast growing organization in the pharmaceutical industry in India. To cater to market requirements, we have dedicated our 6 manufacturing facilities to our core business area – APIs ably supported well-equipped Research & Development laboratory both for process improvement and new product development.
We have been successfully supplying APIs all over the globe for more than Two decades. We are capable of executing extensive API requirements for our customers. Rakshit has an experienced team of skilled employees, who are equipped for API manufacture with adequate care. We have a stand-alone R&D center located in Visakhapatnam with State-of-the-art analytical facilities and around 100 employees. We have wide variety of products in diverse therapeutic segments.
Some of the Active Pharmaceuticals Ingredients (APIs) mentioned in the Rakshit’s product list or brochure or in the product list uploaded in Rakshit’s website may be protected by a valid patent in India or in any other countries concerned. However, mentioning of the name of such product(s) in the product brochure or the product list shall not be construed as infringement in any manner whatsoever. Without prejudice to the foregoing, Rakshit’s supply of API being protected by a valid product patent, if any, is intended solely for uses reasonably relating to the research and development, and regulatory submission under any law for the time being inforce, in India, or in a country other than India that regulates the manufacture, use, sale or import of the said product, strictly under the conditions as set forth in Section 107A of the Indian patents Act, 1970, as amended in 2005, or an equivalent provision (Bolar exemption) thereof in a country other than India. The disclaimer is applicable till the expiration of the corresponding product patent(s), if any, in the respective countries concerned. None of the products will be supplied to the countries wherein this could be in conflict with existing laws. However, the final responsibility lies exclusively with the buyer/end customer